Landfast sea ice in the Bothnian Bay (Baltic Sea) as a temporary storage compartment for greenhouse gases
New publication by N.-X. Geilfus, K. M. Munson, E. Eronen-Rasimus, H. Kaartokallio, M. Lemes, F. Wang, S. Rysgaard, and B. Delille
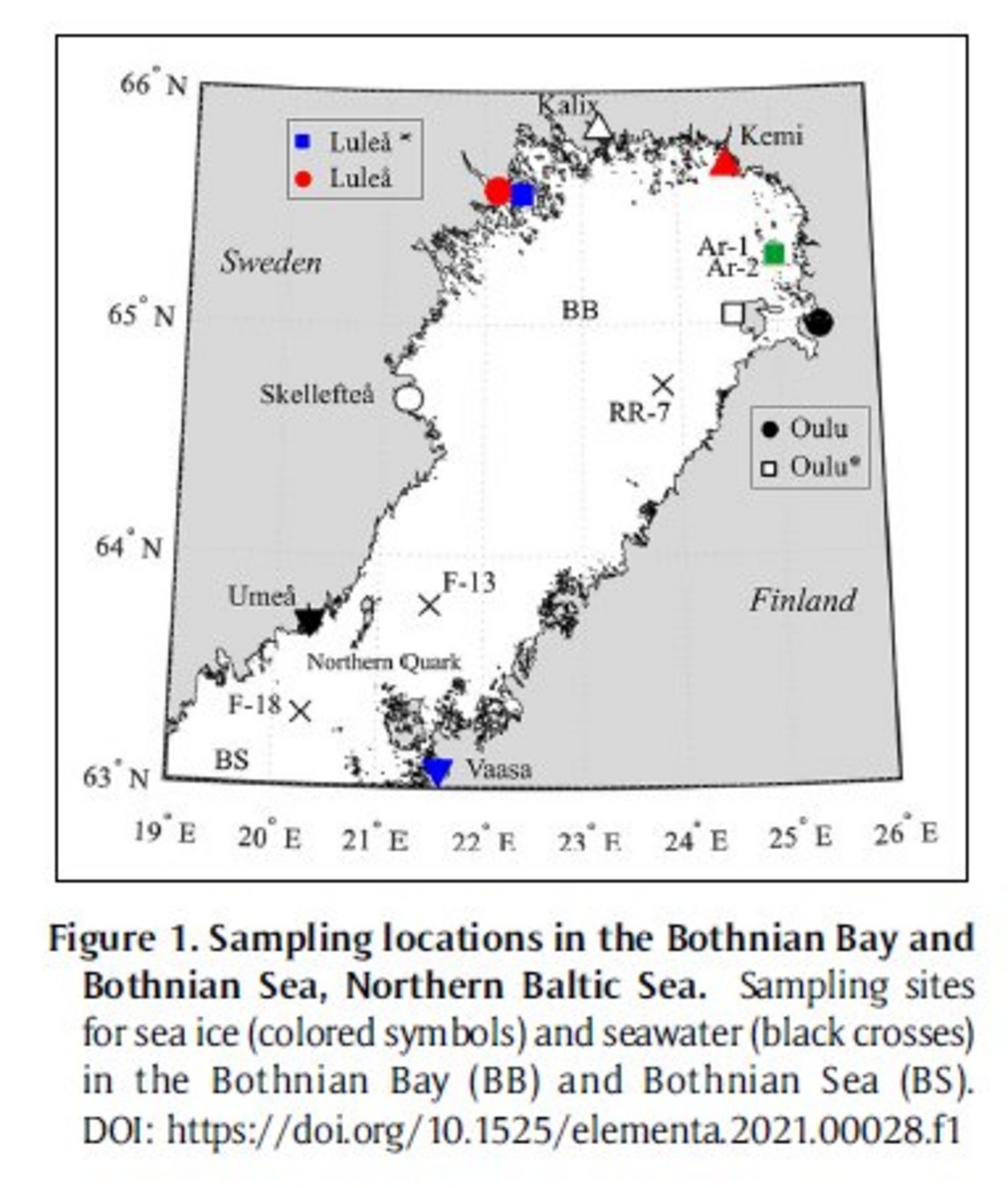
Abstract:
Although studies of biogeochemical processes in polar sea ice have been increasing, similar research on relatively warm low-salinity sea ice remains sparse. In this study, we investigated biogeochemical properties of the landfast sea ice cover in the brackish Bothnian Bay (Northern Baltic Sea) and the possible role of this sea ice in mediating the exchange of greenhouse gases, including carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) across the water column–sea ice–atmosphere interface. Observations of total alkalinity and dissolved inorganic carbon in both landfast sea ice and the water column suggest that the carbonate system is mainly driven by salinity. While high CH4 and N2O concentrations were observed in both the water column (up to 14.3 and 17.5 nmol L–1, respectively) and the sea ice (up to 143.6 and 22.4 nmol L–1, respectively), these gases appear to be enriched in sea ice compared to the water column.This enrichment may be attributable to the sea ice formation process, which concentrates impurities within brine. As sea ice temperature and brine volume decrease, gas solubility decreases as well, promoting the formation of bubbles. Gas bubbles originating from underlying sediments may also be incorporated within the ice cover and contribute to the enrichment in sea ice.The fate of these greenhouse gases within the ice merits further research, as storage in this low-salinity seasonal sea ice is temporary.
