Polyproline type II helical antifreeze proteins are widespread in Collembola and likely originated over 400 million years ago in the Ordovician Period
New publication by Scholl, Connor L. ; Holmstrup, Martin ; Graham, Laurie A. et al.
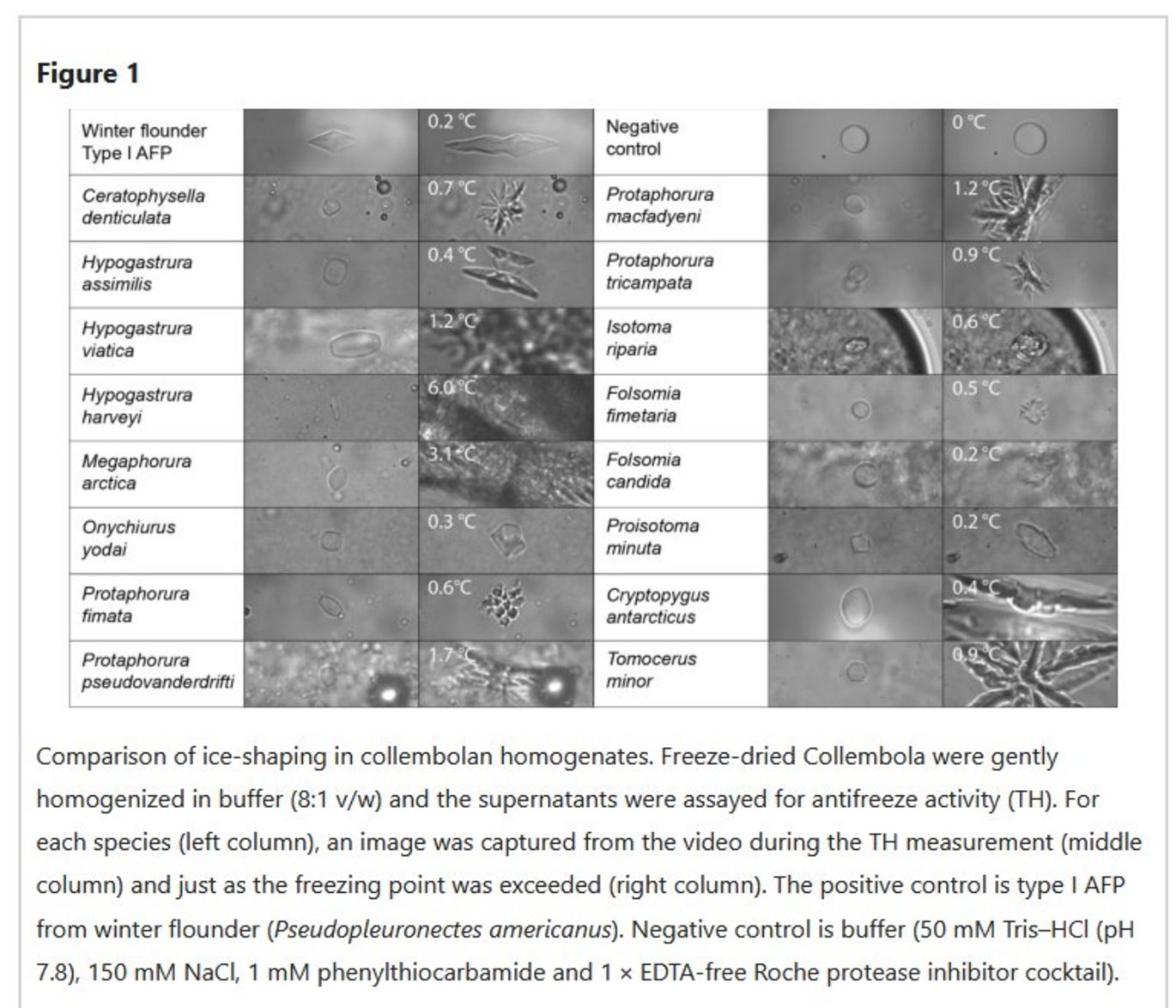
Abstract:
Antifreeze proteins (AFPs) bind to ice crystals to prevent organisms from freezing. A diversity of AFP folds has been found in fish and insects, including alpha helices, globular proteins, and several different beta solenoids. But the variety of AFPs in flightless arthropods, like Collembola, has not yet been adequately assessed. Here, antifreeze activity was shown to be present in 18 of the 22 species of Collembola from cold or temperate zones. Several methods were used to characterize these AFPs, including isolation by ice affinity purification, MALDI mass spectrometry, amino acid composition analysis, tandem mass spectrometry sequencing, transcriptome sequencing, and bioinformatic investigations of sequence databases. All of these AFPs had a high glycine content and were predicted to have the same polyproline type II helical bundle fold, a fold unique to Collembola. These Hexapods arose in the Ordovician Period with the two orders known to produce AFPs diverging around 400 million years ago during the Andean-Saharan Ice Age. Therefore, it is likely that the AFP arose then and persisted in many lineages through the following two ice ages and intervening warm periods, unlike the AFPs of fish which arose independently during the Cenozoic Ice Age beginning ~ 30 million years ago.
