Use, exposure and omics characterisation of potential hazard in nanomaterials
New publication by Ma, NL, Zhang, N, Yong, WTL, Misbah, S, Hashim, F, Soon, CF, Lim, GP, Peng, W & Sonne, C
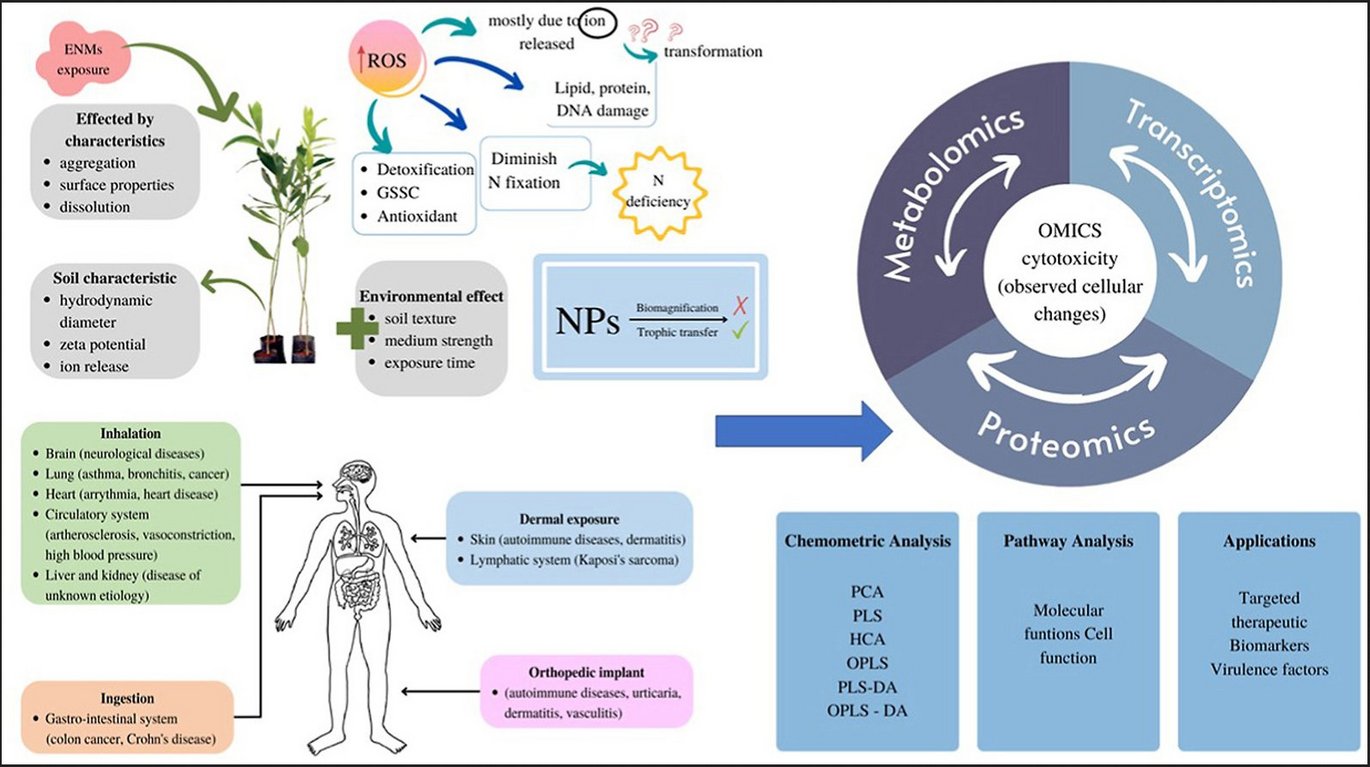
Abstract:
Nanomaterials offer the potential for positive technological impact in a variety of industries. The major breakthrough is in neurological therapeutic applications as their physical and chemical properties allow them to penetrate the blood-brain barrier (BBB). However, questions concerning its safety have arisen as a result of its permeability and the broad application of nanomaterials especially the engineered nanomaterial (ENMs). Due to the large spectrum of ENM properties, pinpointing individual features that caused toxicity is difficult. It is therefore urgent to capitalise on these new developments in ENM safety evaluation. Indeed, novel risk assessment and risk management techniques for humans and the environment across the whole life-cycle of nanomaterial products have emerged in recent years, including systems biology approaches and high-throughput screening platforms. Moreover, the new toxicology technology should practically reduce the number of animal samples required for testing and allow both in vitro and in vivo cell studies. Unlike traditional cytotoxicity, which limits the analysis effect to a single experiment, hazardous risk assessment by integrated omics technologies using high-throughput technologies provides robustness of systemic functional analysis towards ENM, allowing the discovery of biomarkers and functional pathways affecting ENM safety application.
